AutoPlan. Capture requirements right.
A platform built around you
We made sure we have a module for every part of your ServiceNow release process.
View all modules4 min read
Highly regulated industries such as Life Sciences, Pharmaceuticals and Banking, face unique and increasing challenges to deliver change to business stakeholders at rising velocity than ever before. Platforms, such as ServiceNow, are meant to be the answer to traditional problems, but in many cases, they often ask more questions than answer.
We know from our previous blog on DevOps fueling innovation in regulated industries that ServiceNow teams need to plan, create, verify, deploy and monitor with validated tooling, ideally a one-stop-shop for all, and adhere to a strict set of processes across the SDLC. It is not just a case of capturing requirements, building tests and off we go to production, it’s far more complicated.
Today, we can focus in on one element that’s often overlooked, wrongly, that drastically speeds up the verification stage – State Management.
Why is state management important?
ServiceNow customers pursuing real digital transformation require DevOps that’s ready to scale to swiftly, deliver and release new capabilities that are traceable from the original business stakeholder requirements right through to UAT (User Acceptance Testing) sign-off and deployment.
Firstly, let’s tackle what State Management is. At the core of any application, in this instance web based ServiceNow, State Management helps to determine what data is displayed on the screen during the app usage session, when a user interacts with it. In everyday life, this could be as simple as adding products to a shopping cart on an e-commerce store. It’s all possible because a certain set of rules and processes has been adhered to, by you, the user, to enable the ‘state’ to change to ‘in basket’ or similar.
When we take the world of ServiceNow, and specifically delivering change through the Software Delivery Life Cycle (SDLC) in an automated fashion, then ‘States’ can be defined for each entity or artifact within the delivery platform (think, Requirements, Test Plans, Test Scripts). As mentioned above, within heavily regulated industries, it’s not as simple as just creating these entities, and immediately treating them as the gold standard and start using them instantly.
For example, a requirement [entity] may go through several draft layers between the business and technical teams before being considered for development – not uncommon I hear you grumble – the difference is, each of these draft layers (Draft 1, Draft 2 and so on) are treated as ‘State Transitions’ and have a specific set of rules and approvals process enabling the moving of the entity through the State Management Plan.
In regulated industries, each and every change of state must be tracked for auditory purposes and the State management Plan (a simple example can be seen below) must be adhered to for governance and compliance. This process has to be followed for each state to transition, and each entity may have several states:
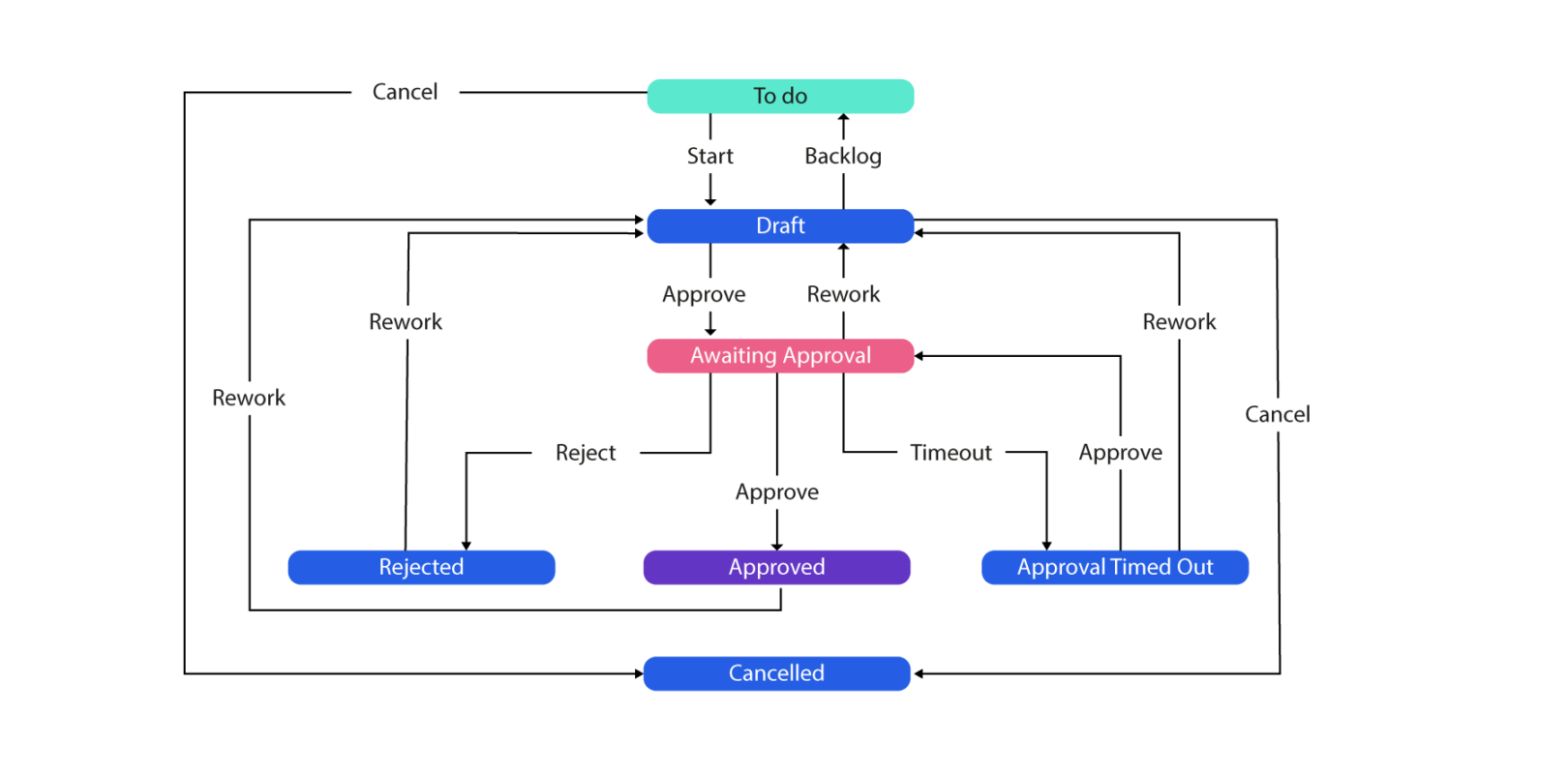
That’s all very well I hear you say, great following of the process, well done team. But what if the process was to wet-ink sign at each step, of each state, for each entity, for every change into the system? Arduous right? We agree…
AutomatePro have seen this process in numerous Pharmaceuticals and Life Sciences where delivery is being slowed down by archaic processes that no longer need to be the normal. This process can not only be automated but improved upon in extreme fashion.
Ultimately, automated (or manual) tests can only be executed in a certain state – an approved one. There is no value running a test that’s known to be out of date (known being the key word here) and this would highlight a great challenge within the delivery teams – change happening outside of the change management process. As time goes on, if processes are not adhered to, the gap only grows…

As the name suggested, automation is in our nature and State Management is no exception. We have created an ‘AutomatePro Repository Instance’ where States and State Transitions can be defined. Rules can be applied to different states, e.g., a record might be read only in the “awaiting approval” state, or a test script only executable in the “Published” state. These states can be linked to a built-in approvals process, linked directly with ServiceNow’s e-signature system (as a scoped app within ServiceNow), to only allow the movement from one state to another with an authorized approval – no more wet ink signatures!
The great thing here is that everything is tracked and traced (thought we got away from that phrase in 2021?) – entities that have transitions through states will have full auditability of entity data history. What this means in practice is a snapshot of the entity which is transitioning state and any of the relationships and related entities for that point in time is taken and stored in the central repository.
The power of having one single pane of glass to view all these states, is that the history of, for example, a requirement record, and its approvals, can be traced right through its process until approved and published – ready to be documented automatically as part of the governance and compliance journey. When using the full AutomatePro suite, these requirements, and their history, can be tracked from cradle to grave, ensuring the change management process is adhered to throughout the SDLC, but also baking in quality right from the requirements capture stage through to delivery to the business.
That requirement can be traced through planning, creating, verifying, deploying and be monitored – ensuring the correct business change (the change that was asked for!) is delivered to the business as quickly as possible, whilst adhering to governance and compliance standards that are absolutely necessary to manage and enable large Life Sciences and BFSI organizations to operate in the world they do.
As the leader in DevOps for ServiceNow, AutomatePro supports Pharmaceutical, Life Sciences, and Banking organisations in their quest to overcome the seemingly impossible task of keeping up with ever-quickening business needs with the added burden ofgovernance and compliance.
To find out how AutomatePro can help power your business and help employees be more productive in their jobs with test automation and documentation creation such as upgrade risk reports, requirements and acceptance documents, or test execution reports, please get in touch by calling us on +44 (0) 20 3473 2986 or email us: hello@automatepro.com