
Create automated tests in seconds.
4 min read
April 6, 2022
Highly regulated industries such as Life Sciences, Pharmaceuticals, and Healthcare face unique challenges and pressures, These are to deliver more and more innovatively than ever before following the COVID Pandemic.
The pandemic has drastically changed the demands on the business, forcing through digital transformation. The importance being placed on business-critical platforms, such as ServiceNow, which is growing at an alarming rate. This increase in productivity, married with a global ServiceNow resource shortage under the constraints of regulatory compliance has ensured that technology is the answer.
ServiceNow Teams need to plan, create, verify, deploy, release and monitor from a single ‘one-stop shop’ that is fully integrated. More specifically, a native ServiceNow DevOps platform that empowers full lifecycle enablement.
How can DevOps relieve compliance pain?
ServiceNow customers pursuing real digital transformation require DevOps that’s ready to scale to swiftly, deliver and release new capabilities that are traceable from the original business stakeholder requirements right through to UAT (User Acceptance Testing) sign-off and deployment.
Typically, large blocks of project time are dedicated to planning and testing to ensure requirements (and tests) can be safely executed to ensure defect-free delivery of changes and the value backlog, a painstaking process with decreasing resources and increasing risk as the amount of change demanded by the business rises.
Where does AutomatePro deliver?
The AutomatePro suite delivers customers a single solution with all key DevOps functions and processes seamlessly integrated. We facilitate the capture of requirements in a structured, human-readable format, with a clear definition of done ready for customer sign-off. These clearly defined requests are automatically created as developer tasks and automated tests, allowing immediate validation through these initial Plan, Code, and Build phases. Underpinned by documentation that consists of requirements specifications, process definition, and unit test reports, we allow customer projects to start with the best, most accurate, and fastest foot forward.
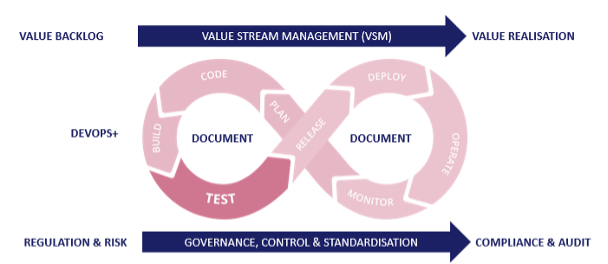
These automatically created business process tests, linked back to the requirements, are added to the regression pack (also incorporating further functional testing, end-to-end integration, and cross-platform testing) and scheduled as quickly as the business demands it. The Automation Core Engine is a scalable and robust architecture to run tests in parallel, radically reducing the time it takes to test (and regression test) any change. These changes are automatically deployed (and alerted if there are any issues) and enter into an automated monitoring process to continuously check on UX, processes, thresholds, and incident creations and ultimately feed information back into the demand funnel.
GxP compliant. GxP shortened to encompass Good Laboratory Practice, Good Clinical Practice, and Good Manufacturing Practice exists to protect patients with standard requirements for the sale of safe products such as drugs, food, medical devices, and cosmetics.
Operating in these industries means high-quality skills are required for implementation, testing, and documentation due to the complicated regulatory implications. This means there is a zero-tolerance policy to comprising quality, yet upholding speed and efficiency is key. This creates a huge challenge for businesses that characteristically result in product delays and poor business performance.
AutomatePro enables customers to ensure quality in both validated and non-validated environments and keep that all-important audit trail, ready for compliance. The opportunity for structured and consistent test execution reporting, coupled with timestamped, full screen, and windowed screenshots at the click of a button allow you to jump between relevant evidence and reporting data in a single repository, quickly and easily. Our native ServiceNow application allows you to group defects, ensure appropriate version control, and view test execution statuses on a dashboard quickly to identify and fix any risks immediately.

Director of ServiceNow Platform at a leading pharmaceutical company articulated that:
“We see AutomatePro as a way to ensure that we can generate consistent results for both our UT (Unit Testing) as well as to ensure we can comply with our Quality Management gates.
Our long term vision is to be able to manage our requirements (both validated and non-validated) within a single repository, perform automated testing to ensure that our technology is compliant with our processes, and be able to generate Quality Management deliverables in an automated fashion (Trace, SIP and SIS) This will allow us to demonstrate the full control that we have over our development and code promotion processes”
AutomatePro’s feature update means businesses can benefit more from enhanced defect management and triage with a defect fingerprinting process and execute securely with crypto vault protection and deliver with full control.
As the leader in DevOps for ServiceNow, AutomatePro supports Pharmaceutical, Life Sciences, and Healthcare organisations in their quest to overcome the seemingly impossible task of keeping up with ever-quickening business needs with the added burden of compliance.
We facilitate releasing at velocity, version control, and the needed quality controls to ensure compliance and ultimately enable our customers to deliver more value through ServiceNow at the speed business users require.
To find out how AutomatePro can help power your business and help employees be more productive in their jobs with test automation and documentation creation such as upgrade risk reports, requirements and acceptance documents, or test execution reports, please get in touch by calling us on +44 (0) 20 3473 2986 or email us: hello@automatepro.com